Abstract
Myelofibrosis (MF) is a heterogeneous disease, displaying marrow fibrosis, and often associated with significant splenomegaly and debilitating symptom burden, marked cytopaenias and an inherent risk of leukaemic transformation. Allo-HCT remains the only curative therapy for MF and is considered in suitable patients with intermediate-II or high-risk disease who have a donor. Previously, it has been reported by Wong et al that MF patients are at significant risk of early hepatotoxicity post allo-HCT which adversely impacted survival (BBMT 2012). The aim of our study was to evaluate risk factors for hepatic biological changes within the first 100 days post-allo-HCT and analyze if the impact on survival.
EBMT affiliated centres were asked to participate in a registry-based study with additional collection of data characterizing liver biological abnormalities and the estimated cause of these abnormalities following MF allo-HCT occurring between 2007 and 2015. Liver biological abnormalities were defined as an abnormal value for at least one of the following parameters: bilirubin, ALT, AST, ALP or GGT anomalies that were classified as Grade 1 adverse event (AE) according to Common Terminology Criteria for Adverse Events (CTCAE) classification occurring within 100 days following allo-HCT. Risk factors for these liver AE were studied using logistic regression. The association of liver AE within 100 days after allo-HCT and overall survival was analyzed using time-dependent Cox proportional hazard models and landmark analyses beginning at day +100.
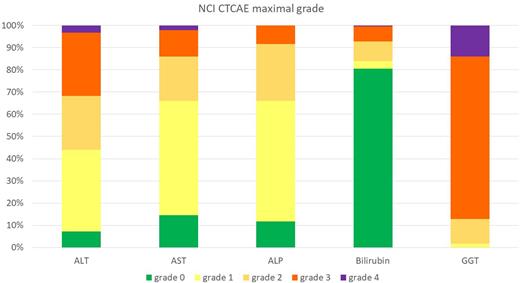
283 patients were included, of whom 171 were male. Median age was 58.7 years (IQR: 22-75). DIPSS score at time of allo-HCT was calculated in 260 patients and was high, intermediate-2, intermediate-1 and low in 20%, 45%, 30%, and 5%, respectively. Reduced intensity (RIC) regimens were utilized in 93% and donor source was a HLA-matched related donor in 26%. Hepatic comorbidities according to the HCT-Comorbidity Index (CI) definition (Blood 2005, 1006: 2912-2919) were reported in 17% of patients prior to allo-HCT. Among the 283 patients, liver AEs occurred in 180 (64%) patients and were associated with the following clinical symptoms/signs: palpable hepatomegaly (n=84), liver pain (n=37), gain of weight (n=96). Figure 1 shows the distribution of maximum bilirubin and liver enzyme abnormalities according to CTCAE criteria during the first 100 days. Increase in bilirubin were most frequently mild (grade 3-4 in 7% of those with liver AE) while increase in GGT were frequently grade 3 or 4 (87%). AE grade 2 or more for bilirubin, AST, ALT or ALP was observed in 127 (45%) patients. Liver AEs were associated with at least one concomitant organ failure in 24% of patients with liver AE: cardiac failure (13%), renal failure (22%) and respiratory failure (16%). Most causes of hepatic AEs were attributed to medications (n=128, including conditioning regimens), sinusoidal obstruction syndrome (SOS) (n=34), hepatic GVHD (n=29), infection (n=14), other (n=2). Among 158 patients assessable on day +100, 70% patients had recovery of liver AEs. Multivariable (MVA) modelling showed poor performance status prior to allo-HCT (<90 vs. ≥90 HR: 2.56, 95% CI 1.28-5.26, p=0.009), higher HCT-CI prior to allo-HCT (3 vs 0 HR: 2.55, 95% CI 1.25-5.42, p=0.01), T cell-depletion (HR: 2.38, 95% CI 1.00-5.78, p=0.05) and prior splenectomy (HR: 2.66, 95% CI 1.03-7.43, p=0.05) increased the risk of liver AE. MVA for OS and NRM were adjusted on age, regimen intensity (RIC versus MAC), Karnofsky score (<90 vs 90-100), pre-graft disease response (untreated, response, no response), type of donor (HLA matched sibling vs other), MF classification (primary, secondary, acute myeloid leukemia at time of HSCT). MVA suggested that any Grade 1-4 early liver AE was not a risk factor for either mortality (HR: 0.90, 95%CI HR: 0.53-1.71) or NRM (HR: 1.06, 95%CI HR 0.65-1.71). Grade 2-4 bilirubin, AST, ALT, ALP was not a risk factor for mortality (HR: 1.27, 95%CI: 0.89-181), neither was SOS (HR: 0.81, 95%CI: 0.35-1.87).
Hepatic AEs occurring early following MF allo-HCT appear very frequent and are usually transient, mostly related to medication exposure, in particular ATG. However, SOS was diagnosed in 34 patients, highlighting that this complication remains a particular issue following MF allo-HCT compared to other myeloid malignancies. Early mild AEs do not appear to impact the overall outcome of MF allo-HCT patients.
Disclosures
Robin:MEDAC, ASTEX: Research Funding. Zuckerman:Orgenesis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BioSight Ltd: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Cellect Biotechnology: Honoraria, Speakers Bureau; Janssen: Honoraria, Speakers Bureau; Novartis: Honoraria, Speakers Bureau. Raj:Jazz Pharma: Speakers Bureau. Kröger:Sanofi: Honoraria; Kite: Honoraria; Neovii: Honoraria, Research Funding; Riemser: Research Funding; DKMS: Research Funding; Amgen: Honoraria; BMS: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Takeda: Consultancy, Honoraria; Jazz: Honoraria. McLornan:JAZZ: Honoraria, Speakers Bureau; CELGENE BMS: Research Funding, Speakers Bureau; NOVARTIS: Honoraria, Research Funding, Speakers Bureau; ABBVIE: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal